Molnupiravir Ivermectin - Molnupiravir Last Of The Small Molecule Coronavirus Hopes Science Aaas
Molnupiravir looks to be a protease inhibitor blocking the cleaving of translated viral polypeptides into functional proteins required for SARS-CoV-2 to replicate successfully. Anyone can make ivermectin.
Moreover the New Jersey-based pharmaceutical company was compelled by a 12 billion procurement agreement to supply approximately 17 million courses of Molnupiravir to the US.
Molnupiravir ivermectin. At the moment you can get the generic version of Mercks Ivermectin for around 45 for a box of eight pills or 79 for a box of 20 pills. Once molnupiravir is approved by the FDA then ivermectin wont be able to get an EUA because an approved treatment will already exist. Mercks New Gamechanger Pill Against COVID Molnupiravir Has Molecular Similarities To Ivermectin.
So I wonder how similar the molecular structure of Molnupiravir is to Ivermectin. Kory for discussions about COVID treatment told me that it seems that molnupiravir is a copy of one of Ivermectins mechanisms This mechanism is to disrupt the SARS-COV-2 virus RNA-dependent RNA polymerase RdRp enzyme said Dr. KENILWORTH NJ Feb.
If you do that math thats around 4 to 7 per pill. Comparing Molnupiravir and Ivermectin What is particularly disturbing is that it appears that molnupiravir contains some of the same molecular qualities as ivermectin which makes you wonder if Merck knows that ivermectin is effective and just sought a more expensive drug that could be marketed as exclusive and new for COVID thereby justifying. Mobeen who runs a medical education center.
Molnupiravir Merck COVIDtreatmentHi everyone. Merck is focusing on molnupiravir. Molnupiravir development codes MK-4482 and EIDD-2801 is an experimental antiviral drug which is orally active and was developed for the treatment of influenzaIt is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine and exerts its antiviral action through introduction of copying errors during viral RNA replication.
The creator of Molnupiravir is the same company that made the controversial drug ivermectin which is used to treat parasitic infections for 300 million people. While the Philippines recently announced a large national ivermectin trial targeting early-onset COVID-19 another commercial study seeks to accelerate sufficient safety and efficacy data in that Southeast Asian nation as well as Merck recently kicked off a Phase 3 trial there testing the oral antiviral drug Molnupiravir led by the Lung Center of the Philippines. On the flipside the US government has made an advanced purchase of 17.
The drug was developed at Emory. However the current data is considered inconclusive. No money to be made.
They include ivermectins original manufacturer Merck which has an antiviral compound molnupiravir in Phase 3 clinical trials for COVID-19. Company scientists continue to carefully examine the findings of all available and emerging studies of ivermectin for the treatment of COVID-19 for evidence of. Top 11 News results.
3 Merck developer of ivermectin has begun late-stage trials for the drug molnupiravir that could actually prevent transmission of COVID-19 file photo. 4 2021 Merck NYSE. The creator of Molnupiravir is the same company that made the controversial ivermectin that is used to treat the parasitic infections of three hundred million people.
Big Pharma sees another win with Mercks upcoming Molnupiravir a drug that has been shown to dramatically reduce the risk of. That might explain the companys recent statement claiming that there is no scientific basis whatsoever for a potential therapeutic effect of ivermectin against COVID-19. Remote viewing is the ability of a human being to perceive information and imagery of remote geographical targets regardless of time and space.
The perspective of Remote Viewing. At the beginning of the pandemic ivermectin was thought to help treat COVID-19. Similar enough to have a positive immediate effect but just dissimilar enough to be qualified unique and worthy of patentalso dissimilar enough to cause problems and.
However if ivermectin was officially recognized as an effective treatment it would legally prevent molnupiravirs EUA until it passes trials and thus delay or endanger the 12 billion deal. I can see using them in conjunction to good. Ivermectin doesnt need an EUA because it passed trials in 1986.
June 10 2021 at 850 am. Merck is also the developer of ivermectin an anti-parasite drug that has gained infamy due to false claims that it could combat the virus - which is the real use for molnupiravir. Lately you would have heard a lot of drama regarding the first of our two pills ivermectin in the news.
That might explain the companys recent statement claiming that there is no scientific basis whatsoever for a potential therapeutic effect of ivermectin against COVID-19. Oct 1 Reuters Positive clinical trial results for Merck Cos experimental antiviral COVID-19 pill reverberated through the healthcare sector on Friday sending the drugmakers. Early in the pandemic ivermectin was thought to help treat COVID-19 however current data are inconclusive.
The Cost of Mercks Ivermectin vs Mercks Molnupiravir Antiviral Pill. It just needs to be recommended to treat COVID-19. MRK known as MSD outside the United States and Canada today affirmed its position regarding use of ivermectin during the COVID-19 pandemic.
Well nowisnt that an interesting question. Lets look at a promising new oral drug Molnupiravir for treating mild to moderate COVID this week. The reason is that Molnupiravir is reportedly very similar to Ivermectin.
Syed Mobeen who hosts a daily medical show and often hosts Dr. Merck lost the patent years ago. Remote viewing or extra sensory perception was developed in the 1970s by the CIA and was used for espionage purposes.
They include ivermectins original manufacturer Merck which has an antiviral compound molnupiravir in Phase 3 clinical trials for COVID-19. Perhaps molnupiravirs merely getting an EUA will have that effect. As you read the news reports on a new anti-viral pill to battle COVID-19 from Merck called Molnupiravir keep in mind Merck is the pharma company that owns the anti-viral pill Ivermectin.

Unitaid Statement Regarding Ivermectin As A Potential Covid 19 Treatment Unitaid
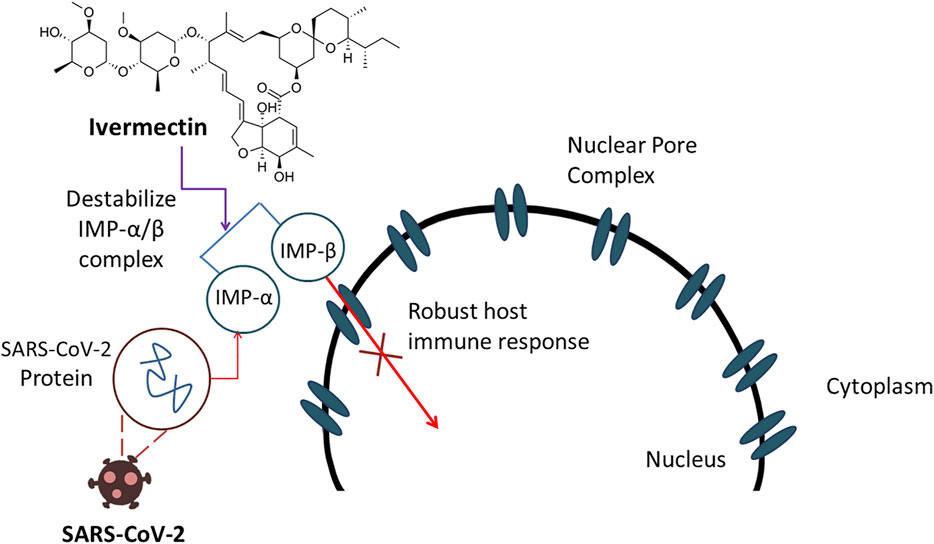
Frontiers Therapeutic Effectiveness And Safety Of Repurposing Drugs For The Treatment Of Covid 19 Position Standing In 2021 Pharmacology

How Molnupiravir Moved To The Head Of The Covid Pill Pack Medpage Today

Minimum Manufacturing Costs National Prices And Estimated Global Availability Of New Repurposed Therapies For Covid 19 Medrxiv

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg
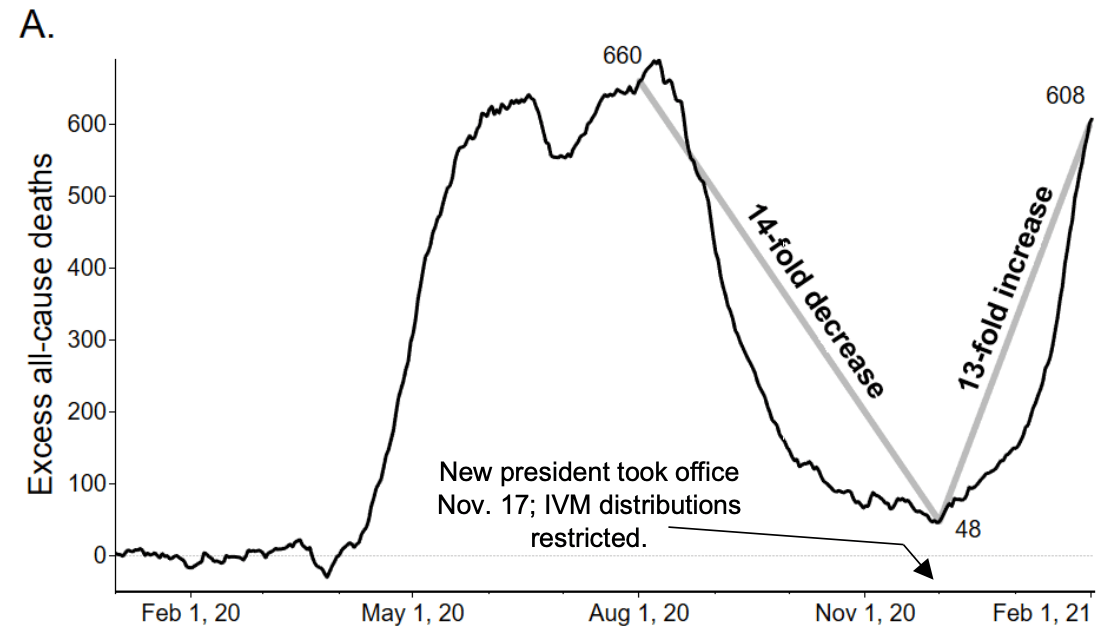
Ivermectin For Covid 19 In Peru 14 Fold Reduction In Nationwide Excess Deaths P 002 For Effect By State Then 13 Fold Increase After Ivermectin Use Restricted

Anti Viral Pill Molnupiravir Shows Promise Against Covid Other Viruses Youtube
Lung Center Qmmc Call For Participants In Molnupiravir Trials Vs Covid 19

Texas A M Expert Warns Against Using Ivermectin To Treat Covid 19 Texas A M Today

Merck Tests Molnupiravir An Investigational Product At The Lung Center Of The Philippines While Large Ivermectin Trial Runs In Parallel

Molnupiravir Last Of The Small Molecule Coronavirus Hopes Science Aaas

:no_upscale()/cdn.vox-cdn.com/uploads/chorus_image/image/69938987/AP21274373443876_copy.0.jpg)







Post a Comment
Post a Comment